Taking a closer look at LHC
The use of the technologies present in particle accelerators, and in particular the advances in the generation of magnetic fields, is the basis of Hadrontherapy (or protontherapy if we refer specifically to the use of protons) as a medical technique.
The use of heavy charged particles (protons and heavy ions - hadrons in general) in radiotherapy allows a completely different dose distribution in tissues than in conventional radiotherapy (see figure), which is its main advantage over photon radiotherapy. When these particles penetrate the body, their energy loss per unit length is inversely proportional to the square of their velocity. This dependence means that the maximum energy loss and the highest ionisation density occur at the end of the particle's trajectory (BRAGG peak), when its velocity is close to zero.
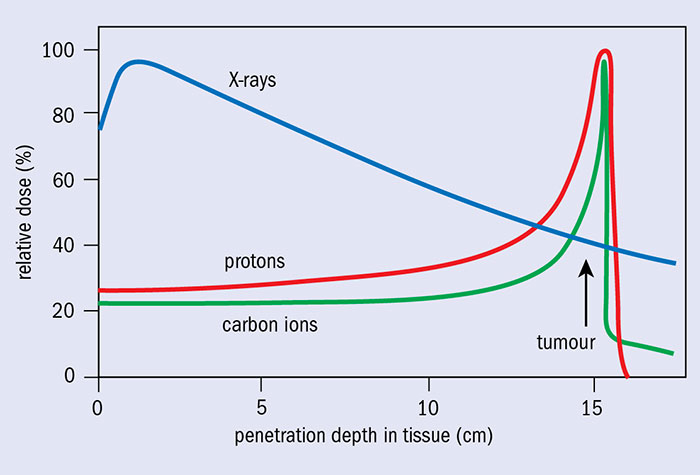
The Bragg peak (Imagen: CERN Courier)
The energy loss rate of a monoenergetic beam when passing through a uniform medium is always the same, so the particles in the beam will slow down to the same depth, which is called the particle range. Therefore, the depth of the Bragg peak depends on the initial energy of the protons and the tissues they pass through. By modulating the energy of the incident particles, it is possible to extend the region of the Bragg peak to localise the dose over a larger volume. So, to ensure a uniform dose throughout the tumour volume, it is necessary to superimpose several Bragg peaks of different energy, i.e. to launch protons that will deposit their energy at different depths in the tumour. This is called Spread Out Bragg Peak (SOBP).
In addition, the radiation dose decreases sharply behind the Bragg peak, which prevents critical organs and healthy tissue from receiving.
These physical characteristics of the proton and ion beams make this type of radiotherapy particularly suitable for deep tumours or tumours close to critical structures in the body, as the dose delivered to the healthy tissue surrounding the tumour is considerably lower than that delivered by conventional radiotherapy.
There are two main treatment techniques: passive scattering ('single/double-scattering') or active scattering ('beam-scanning'). Passive scattering systems use materials that allow the beam to be scattered in its transverse direction until a uniform dose is achieved across the width of the tumour. However, in active systems, taking advantage of the fact that protons (hadrons) are charged particles, a magnetic deflection system is used to move the beam vertically and horizontally until it covers the entire surface of the tumour. It was in 1997 that the active system was first developed at GSI (Helmholtzzentrum für Schwerionenforschung, Germany) and PSI (Paul Scherrer Institute, Villingen, Switzerland). Since then, and taking all the innovations, improvements and optimisations in the field of particle accelerators, and in particular in the area of magnetic fields, great advances in Hadrontherapy have been achieved.
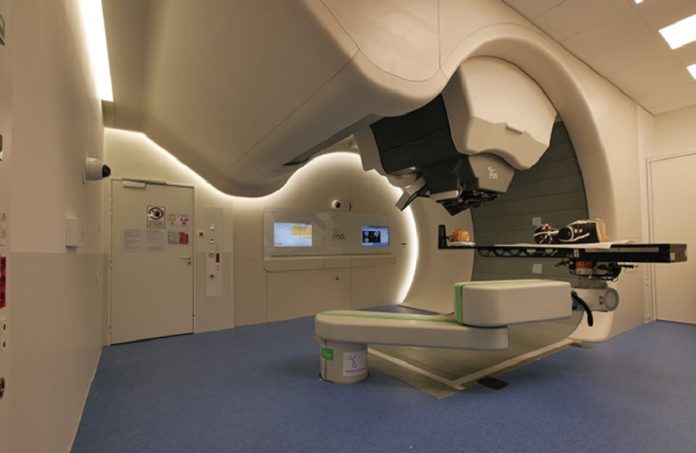
Gantry System (Imagen: CERN Courier)
An outline of a proton therapy (hadrontherapy) area can be presented as follows:
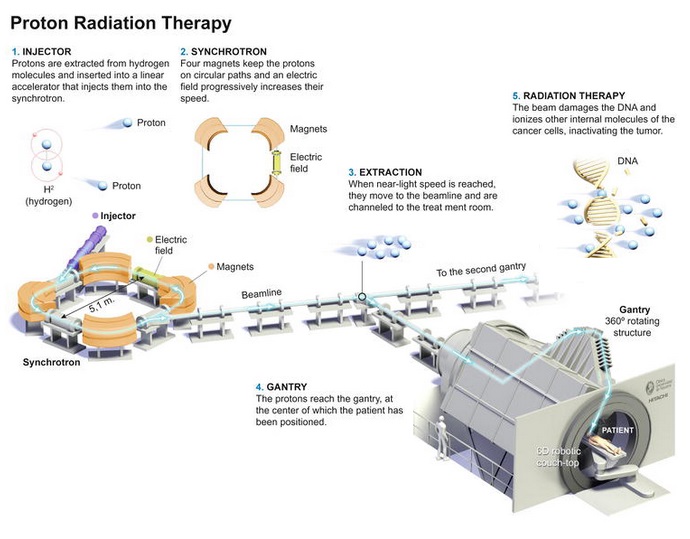
This image is taken from: Calvo Manuel F. A. et al (2020). Proton Cancer Therapy: Synchrotron-Based Clinical Experiences 2020 Update. From the Edited Volume Proton Therapy Current Status and Future Directions Edited by Thomas J. FitzGerald and Maryann Bishop-Jodoin. DOI: 10.5772/intechopen.94937
A novel contribution from CERN is GaToroid, a lightweight, superconducting toroid that can surround a patient and potentially revolutionise the delivery of hadrons for various therapies.
In Spain, 10 Hadrontherapy centres are in the development phase for the public system. In particular, the Proton Therapy Centre in Galicia will be a European benchmark. It is expected to be operational in 2025. In addition to having an area for the treatment of patients, it will have an area dedicated exclusively to research.
More information:
https://enlight.web.cern.ch/what-is-hadron-therapy
https://cerncourier.com/a/cern-takes-next-step-for-hadron-therapy/
https://cerncourier.com/a/proton-therapy-enters-precision-phase/
https://home.cern/news/news/knowledge-sharing/clinical-trials-using-carbon-ions-begin-cnao-0
|
AUTHORS Xabier Cid Vidal, PhD in experimental Particle Physics for Santiago University (USC). Research Fellow in experimental Particle Physics at CERN from January 2013 to Decembre 2015. He was until 2022 linked to the Department of Particle Physics of the USC as a "Juan de La Cierva", "Ramon y Cajal" fellow (Spanish Postdoctoral Senior Grants), and Associate Professor. Since 2023 is Senior Lecturer in that Department.(ORCID). Ramon Cid Manzano, until his retirement in 2020 was secondary school Physics Teacher at IES de SAR (Santiago - Spain), and part-time Lecturer (Profesor Asociado) in Faculty of Education at the University of Santiago (Spain). He has a Degree in Physics and a Degree in Chemistry, and he is PhD for Santiago University (USC) (ORCID). |
CERN CERN Experimental Physics Department CERN and the Environment |
LHC |
IMPORTANT NOTICE
For the bibliography used when writing this Section please go to the References Section
© Xabier Cid Vidal & Ramon Cid - rcid@lhc-closer.es | SANTIAGO (SPAIN) |